There are several sources of free printable periodic table templates online. The periodic table is a graphical representation of the elements and their properties, arranged by time and by date of discovery. The first manmade element was “T” and is represented by the letter “T”. The table also shows how electrons fill the shells of atoms. Some sources offer both PDF and image files. Both types of files are customizable, meaning that you can print as many copies as you like.
Sections of Periodic Tables:
Molar mass
What is the molar mass of an element? In the periodic table, the mass is a number on the bottom of the square. There is a decimal point after the mass, but some masses are in parentheses. These masses are unknown and are dangerous or rare elements. In the upper left corner, the element’s atomic number is given, as is its molar mass. The middle letter is the element’s atomic symbol, which can be a single or double letter.
Oxidation states
The periodic table has oxidation states for each element. As the number of atoms increases, the covalency of an element decreases. In the case of the first eight elements, this decreases to zero and helium reaches a state of +4 only with a large negative potential. The rest of the elements are in between. In Table 1-1, the elements in groups A, B, and C have coordination numbers of 4 or 6. For a more detailed explanation, refer to Chapter 12.
Groups
If you want to learn more about the elements, then you can use the printable periodic table. The periodic table lists 118 recognized elements by the International Union of Pure and Applied Chemistry (IUPAC). It also shows when an element was discovered and which year they were officially named. Each element is represented by a specific symbol, which indicates how many electrons it possesses. The periodic table also shows the filling of electron shells.
Free Printable Periodic Table Templates:

Here’s the download button for this Periodic Table Template Vol-01.
The download button for this Periodic Table Template Vol-02 is here.

Here’s the download button for this Periodic Table Template Vol-03.
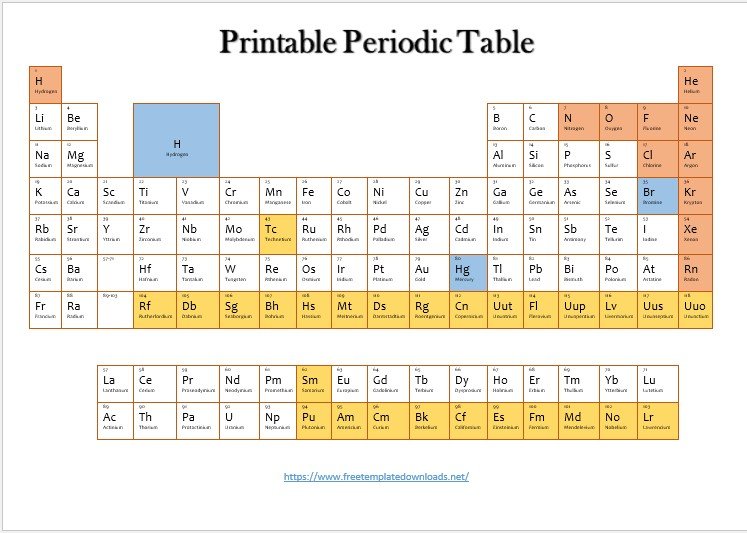
The download button for this Periodic Table Template Vol-04 is here.

Here’s the download button for this Periodic Table Template Vol-05.

The download button for this Periodic Table Template Vol-06 is here.
What Actually a Periodic Table?
A Periodic Table is a scientific table that consists of rows and columns. The columns represent the groups while the rows represent the periods. The rows and columns are then filled with elements that correspond to the various groups and periods indicated. To understand a Periodic Table, you need to know the number of electrons that an atom has. A typical Periodic Table consists of 8 groups and about 4 periods. To understand this, you need to know the basic structure of an atom.
An atom consists of a nucleus containing protons (which are positively charged), and energy levels containing electrons (which are negatively charged). The nucleus is the center-most part of the atom. The number of energy levels depends on the number of electrons present in the atom. The first energy level can hold only two electrons while all the others can hold up to 8 electrons. Therefore, if an atom has 10 electrons, then its electron configuration will be 2.8. This means that it will have two energy levels and 8 electrons on its outermost energy level.
Example of Atoms:
Using the example given above, we can conclude that the said atom is in group 8 and period 2. This is because the group is determined by the number of electrons on the outermost energy level, while the period is determined by the number of energy levels present. All the elements in group 7 of the Periodic Table are known as halogens. The elements in group 8 of the Periodic Table are known as noble gases. Hydrogen has only one electron. Therefore it can either be in group 1 or group 7 of the Periodic Table. However, most of the time, Hydrogen always appears in group 1 of the Periodic Table.
Groups of the Periodic Table
- Group 01 Elements: Group 1 of the Periodic Table consists of metals. These are the elements that have only 1 electron on their outermost energy level. For them to gain stability, they need to lose this outermost electron. The elements of this group can easily bond with the elements in group 7 of the Periodic Table. The elements in group 1 are always highly reactive, and they form strong metallic bonds when bonding.
- Group 02 and 03 Elements: Group 2 and three elements are also metals. They have to lose 2 and 3 electrons respectively for them to become stable.
- Group 04 Elements: Group 4 elements are called transition metals. This is because they are neither metals nor non-metals. For them to become stable, they can either lose the 4 electrons on their outermost energy level or gain 4 more electrons.
- Group 05 and 06 Elements: Groups 5 and 6 are non-metals, and they have to gain 3 and 2 electrons respectively for them to become stable.
- Group 07 Elements: Group 7 elements are called halogens and they gain 1 electron to become stable, while group 8 elements are called noble gases or rare gases.
The number of energy levels corresponds to the period of the element. For example, if the electron configuration is 2.8.2, then the element has 3 energy levels and thus belongs to period 3 and group 2 of the Periodic Table.



